Evaluation of Recovery of Basic Analytes Using a Mixed-Mode Strong Cation Exchange SPE Microplate by HPLC-MS
Jun 01 2022
Author: James Edwards on behalf of Porvair Sciences
Free to read
Articles are free to download. Unlock the article to be shown more content, graphs and images.
Sample preparation is important in any type of chromatography analysis. While it can add on extra time, the process of cleaning up samples before injection onto a system result in a range of benefits to the analyst - better recoveries, more reproducible analysis, less downtime of instruments, reduction of troubleshooting, as well as less complex chromatograms due to the reduction of unwanted compounds being injected. All of these can result in time saved which could be needed for repeated work or maintenance on instruments.
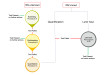
Traditional SPE products consist of a loose-filled resin sandwiched between two frits. While this is known to work, it can come with some problems which can complicate analysis or result in poor data being produced. These problems are a result from how the product is packed into a well or cartridge - voiding can occur under the top frit; channels could form through the resin bed which can cause less efficient interactions between the resin and the analyte(s) or there could be variation on compression or resin weight that was dosed into each product.
图1。与松散填充SPE方法相关的常见问题。
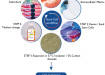
Microlute®CP SPE产品包含一种独特的混合设计,均匀分布的固体互联网络与保持介质相结合。这种设计的优点是通过产品的流动是一致的,并增加了分析物和结构中存在的保留介质之间的相互作用。这两个特点结合在一起,产品具有高回收值和可重复性结果。This technical note uses the 30 mg Microlute® CP Strong Cation Exchange (SCX) 96 well plate to compare performance in recoveries and reproducibility against five competitor loose-filled 30 mg SCX products.
Figure 2. Schematic of the hybrid polymeric structure showing the porous structure of the frit with the active resin immobilised throughout the pore structure.
Introduction
Microlute®CP SCX产品是一种混合模式聚合物SPE产品-离子交换和反相的组合。这就产生了一个具有两种保留机制的产品,可以对其进行微调,以使SPE方法具有更大的灵活性。反相功能允许分析物在疏水相互作用上分离,允许保留被有机改性剂浓度改变。而离子交换允许树脂和带电分析物之间选择性强离子相互作用。在SPE中引入聚合物树脂比使用硅基树脂[1]带来了一些额外的优势。These include:
• Polymeric structures do not contain any of the highly active sites found in silica. These include silanol groups which can cause unwanted secondary interactions with analytes which in some cases could cause irreproducible recoveries.
• Silica’s structure is also very susceptible outside of the pH range of 2 - 7.5. If pH is outside this range, hydrolysis of the silica or bonded functional group on the surface could occur [2]. This will result in very poor and irreproducible recoveries. On the other hand, polymeric resins are resistant to pH allowing them to work over the whole pH range (pH 0 – 14).
连接到二氧化硅表面的官能团需要用有机溶剂来激活保留机制,然后用水溶液平衡。而聚合物树脂则不需要经过这个条件处理步骤。
• Polymeric resins are less sensitive to drying out during the SPE process where silica resin can become dry and lose their retentive function [3].
Strong ion exchange does not work well for strongly acidic or basic analytes (analytes which have a charge over the whole pH range) due to the irreversible binding of charged analyte to the charged ion exchange resin. Weak analytes do work well with strong ion exchange. This is because it is possible to turn the analyte’s charge on or off for a weakly acidic or basic analyte allowing selective binding with a change of pH. To optimise this binding, the 2 pH rule is applied:
• When the pH of the solution in which an analyte is dissolved is equal to the pKa value of the analyte, the analyte is 50% ionised, pH can then be used to adjust how ionised a compound is:
◊ Acidic: 100% ionised: 2 pH above pKa or 100% un-ionised: 2 pH below pKa
◊ Basic: 100% ionised: 2 pH below pKa or 100% un-ionised: 2 pH above pKa
Figure 3. A diagram to show the 2 pH rule for a weak acid compound and weak basic compound, with a pKa of 4 and 8, respectively.
For a typical strong ion exchange method, analytes are pre-treated to a pH where they are charged and then loaded onto the resin. This allows them to bind strongly to the resin with the ionic interactions. The same pH will be maintained on the wash steps of the method allowing the analyte(s) to keep binding to the resin while washing off interfering com- pounds. To allow elution, pH is changed to neutralise the charge allowing the analyte(s) to stop binding to the resin and elute from the product.
Experimental
表1。高效液相色谱条件。
LC system Agilent LC-MS, (With a 1260 LC
and Single Quadrupole Mass Spectrometer)
Column Raptor Biphenyl 30 x 2.1 mm, 1.8 µm
Column temp. 45°C
Injection volume 2.00 µL
Flow rate 600 µL/min
Mobile phase A 0.1% Formic acid in water
Mobile phase B 0.1% Formic acid in methanol
Solvent Composition Time (min) A% B%
0.10 95.0 5.0
4.30 57.5 42.5
6.50 57.5 42.5
6.51 20.0 80.0
8.20 20.0 80.0
8.21 95.0 5.0
14.00 95.0 5.0
Table 2. Mass Spectrometer Conditions.
Parameter Value
Gas Temperature 350°C
Gas Flow 13 L/min
Nebulizer 30 psi
Capillary Voltage 4000 V
Dwell Time 100 V
Fragmentor Voltage SIM
Scan Type ESI
Ion Mode ESI
Chemicals
Caffeine, salbutamol, procainamide, atenolol, pindolol, propranolol, desipramine, protriptyline, imipramine, amitriptyline, nortriptyline, formic acid, methanol, water, 35% ammonia solution.
Sample Preparation
A stock of 1,000 µg/ml of all the basic analytes was made in methanol. A basic load solution was made by diluting 500 µL of the stock solution to 50 mL with water containing 0.1% (v/v) formic acid.
Solid Phase Extraction Method
对于Microlute®CP SCX产品和竞争产品,共测试了12口井。每个测试井用1000µL甲醇调节,然后用1000µL水平衡。然后将1000µL的基本加载液完全加载到板上。装上后,取1,000 L含0.1% (v/v)甲酸水溶液的水清洗吸附剂。然后用0.1% (v/v)甲酸溶液在甲醇中进行强力有机洗涤。
用含5% (v/v)氨的甲醇500µL进行洗脱。然后用Porvair Sciences ultrav®Levante(# 500226)在氮气下35°C蒸发到干燥。另用500µL含5% (v/v)氨的甲醇重复洗脱,同样方法蒸发至干燥。
为了重建每个样品,在每个收集瓶中加入1000 μ g/ml咖啡因(ISTD)的10 μ L,然后加入60% (v/v)甲醇/水,含0.1% (v/v)甲酸的790 μ L,得到12.5 μ g/ml溶液。12.5µg/mL溶液40µL,加入60% (v/v)甲醇/水,0.1% (v/v)甲酸的760 uL进一步稀释,得到0.625µg/mL溶液可供注射。
Results and Discussion
图4。基础分析物的色谱校正标准品。表3中可以找到峰值分配。
表3。分析的基本化合物的性质和质谱参数-来自Pubchem[4]的预测值。
No. Compound Type R.T
(min) Formula Molecular Mass LogPa pKaa
1 Salbutamol Basic 0.70 C13H21NO3 239.31 0.3 10.3
2 Procainamide Basic 1.04 C13H21N3O 235.33 0.9 9.3
3 Atenolol Basic 1.23 C14H22N2O3 266.34 0.2 10.4
4 Pindolol Basic 3.09 C14H20N2O2 248.32 1.8 9.3
5 Caffeine ISTD 3.71 C8H10N4O2 194.19 -0.1 14.0
6 Propranolol Basic 5.56 C16H21NO2 259.34 3.0 9.4
7 Desipramine Basic 6.87 C18H22N2 266.40 4.9 9.6
8 Protriptyline Basic 6.98 C19H21N 263.40 4.4 9.7
9 Imipramine Basic 7.09 C19H24N2 280.40 4.8 9.4
10 Nortriptyline Basic 7.32 C19H21N 263.40 3.9 10.5
11 Amitriptyline Basic 7.57 C20H23N 277.40 5.0 9.4
Recovery Comparisons
Figure 5. Analyte recovery comparisons against other commercial SPE products.
Reproducibility Comparison
Figure 6. Analyte %RSD comparisons against other commercial SPE products.
For reproducibility, a lower %RSD means the recovery was more reproducible. The analyte’s %RSD values can be seen in Figure 6. The Microlute® CP SCX managed to maintain a %RSD value of less than 2.6% for every compound analysed. It outperformed every competitor for reproducibility on each compound with only amitriptyline being closely matched. There was no issue of irreproducible results for either the most extreme hydrophilic compound or the most hydrophobic. This again shows that the Microlute® CP SCX product is performing excellently across a wide range of basic analytes.
Summary
The Microlute® CP SCX 30 mg 96 well plate can effectively retain a wide range of hydrophilic and hydrophobic basic compounds. It offers advantageous recoveries across the range of different classes of analytes. Lower %RSD values are seen when comparing against competitor plates for every compound analysed in this study - 1.6%RSD on average for the Microlute® CP SCX compared to the 2.4%RSD (best competitor) and 5.9%RSD (worst competitor). This ensures the product gives reliable and reproducible results which is an important metric in testing where confidence in the data output is required.
References
1. C. F. Poole, Solid-Phase Extraction, Elsevier, 2020, p. Chapter 3: Porous polymer sorbents.
2 P. V. Brady and J. V. Walther, “Controls on silicate dissolution rates in neutral and basic pH solutions at 25°C,” Geochimica et Cosmochimica Acta, vol. 53, no. 11, pp. 2823-2830, 1989.
3. M. N. Qureshi, G. Stecher, C. Huck and G. K. Bonn, “Preparation of polymer-based sorbents for solid phase extraction of polyphenolic compounds,” Central European Journal of Chemistry, vol. 9, no. 2, pp. 206-212, 2011.
4 “PubChem,” National Library of Medicine, [Online]. Available: https://pubchem.ncbi.nlm.nih.gov/. [Accessed 25 04 2021].
Free to read
Articles are free to download. Please login to read this article or create an account.
Digital Edition
Lab Asia 29.4 - August 2022
2022年8月
In This Edition Chromatography - Automated Sample Preparation:The Missing Hyphen to Hypernation - New Low Volume Air Sampler for PFAS Analysis - Analytical Intelligence Starts with the Samp...
View all digital editions
Events
ACS National Meeting & Expo, Fall 2022
Aug 21 2022Chicago, IL, USA & Online
Aug 22 2022Frankfurt, Germany
Aug 27 2022Maastricht, Netherlands
Aug 28 2022Lisbon, Portugal
Aug 31 2022Singapore