-
 Fine Cut have extensive experience and expertise in producing durable labels and overlays.
Fine Cut have extensive experience and expertise in producing durable labels and overlays.
Durable Medical Labels for the Medical Industry
Mar 10 2022
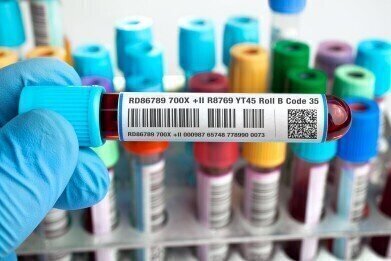
Fine Cut Group are a leading UK based manufacturer ofdurable, innovative labelsfor the medical industry, including medical device labelling, clinical trial and sample identification labels and pharmaceutical labels. The company has worked side by side with the NHS, Government bodies and private laboratories providing label solutions for over 30 years.
The team at Fine Cut have extensive experience and expertise in producing durable labels and overlays catering for a broad spectrum of medical, laboratory and pharmaceutical equipment. Manufacturing for both short and long run batches the company are able to provide recommendations for a variety of medical label applications ranging from patient monitoring equipment to analytical instruments. Durable labels to the latest UL969 guidelines can also be manufactured.
Fine Cut Group’s medical labels are manufactured from high quality materials including polyester, polycarbonate and polypropylene sourced from 3M, Avery, Ritrama and Lintec to name a few. The range of premium label materials offer you a durable and reliable method for identifying a range of medical equipment or samples used within the laboratory.
Fine Cut Group offers a full in-house bureau service, (via our ISO 9001 work frames) facilitating all print requirements including QR codes, 2 data matrix codes and barcode variable data with 100% scanning of all barcodes and 2D matrix codes. Labels are suited for extreme environments including freezer storage -20c to -196c and have enhanced resistance to a range of solvents, chemicals and handling ensuring that all print remains legible (not smudged).
Choose Fine Cut Group and its wealth of experience to be your reliable partner for all of youmedical and Laboratory labellingneeds, the company will deliver quality on time products for your every requirement.
For further information or technical guidance please get in touch today.
Digital Edition
Lab Asia 30.2 - April 2023
April 2023
在这版——On-Colum色谱文章n Sample Focussing: a Personal Perspective Mass Spectrometry & Spectroscopy Articles - Multi-scale, multi-modal and operando imaging wi...
View all digital editions
Events
Apr 30 2023Dublin, Ireland & Online
Apr 30 2023Denver, CO, USA
May 09 2023Hannover, Germany
May 10 2023Beijing, China
May 14 2023Basel, Switzerland







.jpg)











